Greetings from Freyr South Korea
Freyr is a leading Regulatory solutions provider offering comprehensive services to life sciences firms across the regulatory spectrum. With a robust presence spanning over 120 countries, we facilitate the "local to Global" journey for life sciences companies.
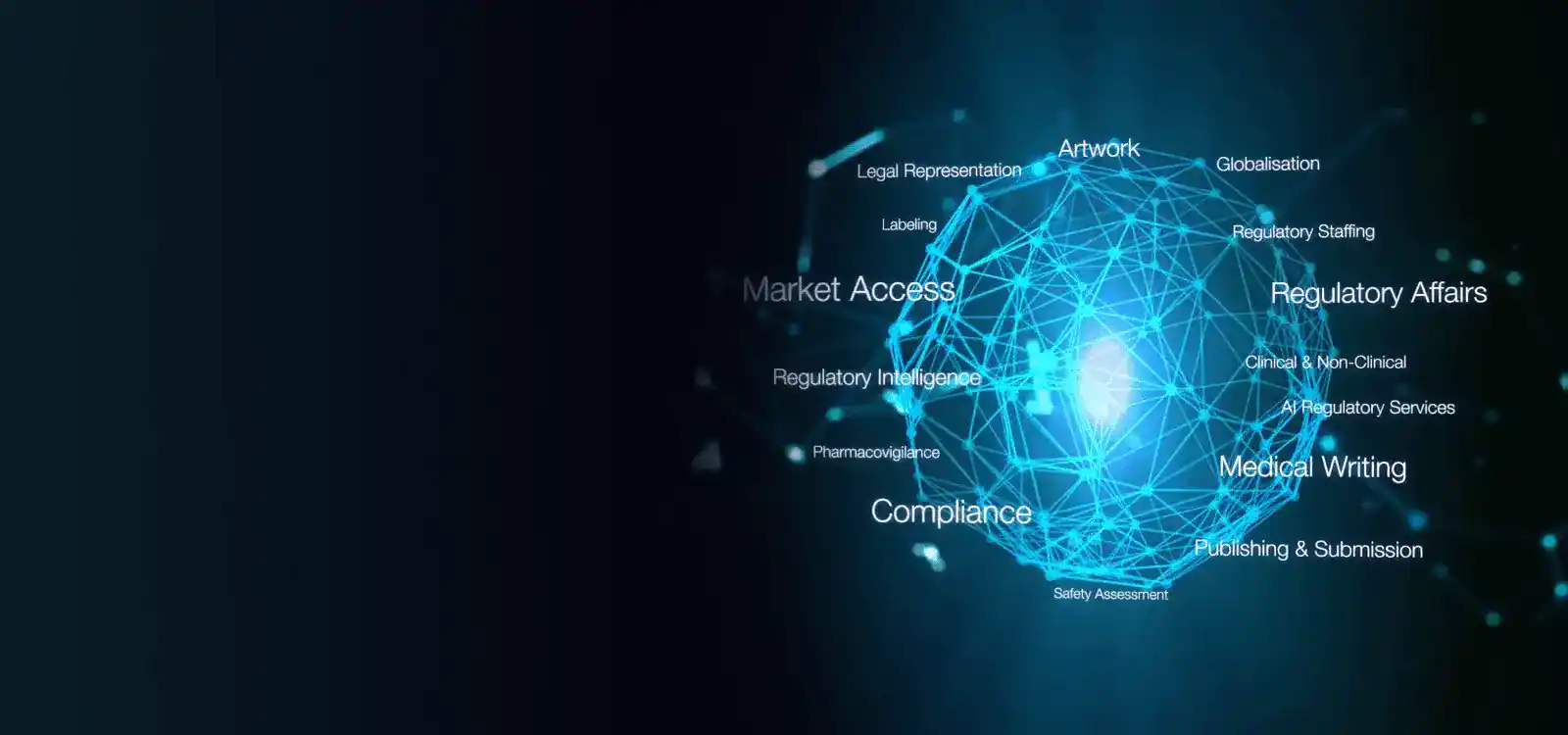
In South Korea, Freyr Korea plays a pivotal role in assisting companies with product registrations under the Ministry of Food and Drug Safety (MFDS). Our services encompass Regulatory Strategy formulation, Market Intelligence, In-country representation, Post-Market Surveillance (PMS) functions, and more. Additionally, we provide cutting-edge regulatory software solutions tailored to streamline the entire MFDS registration life cycle.
Global Presence in
25+ Countries2500+
Inhouse Regulatory
Experts & GrowingSupport in
120+ Countries2100+ Global
clients & growing







 Medicinal Products
Medicinal Products Medical Devices
Medical Devices Consumer
Consumer