
Pharmaceutical businesses looking for new business prospects find South Korea to be an attractive market with a fast-increasing pharmaceutical industry. With over nine-hundred (900) new drugs in the pipeline and exports totalling USD 3.1 billion, South Korea provides enormous potential for big pharma manufacturing.
In South Korea, submitting a New Drug Application (NDA) or Biologics License Application (BLA) is a critical step in bringing new pharmaceuticals to market. The Ministry of Food and Drug Safety (MFDS) is in-charge-of reviewing and permitting NDAs and BLAs in South Korea. The review procedure can take up to one-hundred-and-eighty (180) days, but if the drug is designated as a priority review or fast-track drug, it may be shortened.
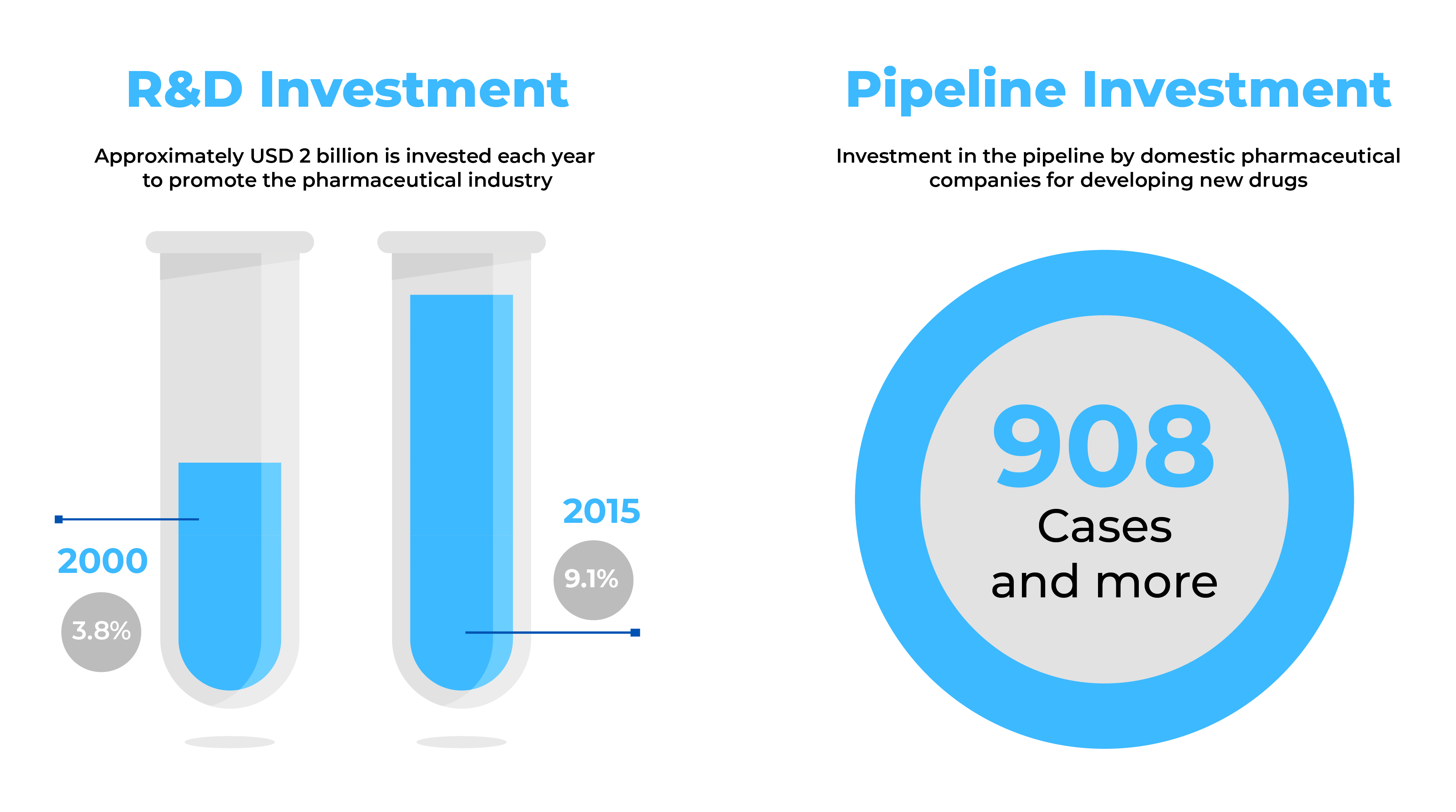
Source: Health Industry Trends-September 2014 & Global data (2017)
The NDA/BLA processes in South Korea offers advantages such as a quicker approval process, clear regulations and requirements, incorporation of Real World Evidence (RWE), and exemptions for orphan drugs. However, the NDA/BLA process in South Korea can be complex and time-consuming. The process of submitting a New Drug Application (NDA) or Biologics License Application (BLA) in South Korea involves several steps.
Here is an overview of the process:
- Preclinical Testing: Before a drug can be evaluated in humans, it must first be tested in animals to ensure its safety and efficacy. Typically, this testing is carried out on animals.
- Clinical Trials: Following the completion of preclinical research, the medicine advances to human clinical trials. These studies are divided into three (03) phases, each with a bigger number of participants.
- NDA/BLA Submission: Following the completion of clinical studies, the sponsor may submit an NDA or BLA to South Korea's Ministry of Food and Drug Safety (MFDS). All five (05) electronic Common Technical Document (eCTD) modules, which cover non-clinical, clinical, and Chemistry, Manufacturing, and Controls (CMC) data, must be included in the application.
- Approval: If the MFDS determines that the medicine is safe and effective, the NDA/BLA will be approved. The medicine can be advertised and sold in South Korea once it has been approved.
Still doesn’t answer all your questions?
Let the experts clarify.
To gain a detailed perspective on NDA/BLA processes in South Korea, Freyr has scheduled a free webinar with our experts from South Korea. Join us for the “Overview of the NDA/BLA Process in South Korea”, scheduled on November 29, 2023,” at 11:30 AM IST (ROW) and at 10: 00 AM EST |3:00 PM GMT |4:00 PM CET (Americas & EUA). Register Now! Stay safe. Stay informed.


